Multiple Choice
Three or four of the following illustrations depict different structural isomers of the organic compound with molecular formula C₆H₁₄.For clarity,only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted.Which one,if any,is not a structural isomer of this compound?
A) 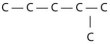
B) 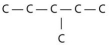
C) 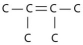
D) 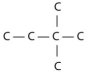
E) Each of the illustrations in the other answer choices depicts a structural isomer of the compound with molecular formula C₆H₁₄.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which two functional groups are always found
Q15: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -The figure above
Q35: Organic molecules with only hydrogens and five
Q38: Why are hydrocarbons insoluble in water?<br>A) The
Q40: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which of the
Q55: Organic chemistry is currently defined as<br>A) the
Q57: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which molecule shown
Q63: Which of the following statements correctly describes
Q66: Which action could produce a carbonyl group?<br>A)
Q79: Choose the term that correctly describes the