Multiple Choice
The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
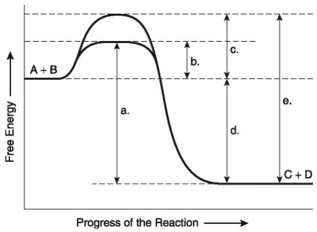
-Which of the following terms best describes the forward reaction in the figure above?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
E) chemical equilibrium, ∆G = 0
Correct Answer:

Verified
Correct Answer:
Verified
Q18: A solution of starch at room temperature
Q32: According to the induced fit hypothesis of
Q42: A system at chemical equilibrium<br>A) consumes energy
Q49: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" Rate of
Q64: During a laboratory experiment, you discover that
Q72: The following questions are from the end-of-chapter
Q73: Succinate dehydrogenase catalyzes the conversion of succinate
Q76: Which of the following is an example
Q83: Use the following information to answer the
Q84: The following questions are based on the