Multiple Choice
For the following SN2 reaction, what would be the relative rate of reaction if the concentrations of the alkyl bromide and potassium acetate (CH3CO2K) were tripled?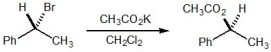
A) No change in the relative rate
B) The relative rate will increase by three times
C) The relative rate will increase by six times
D) The relative rate will increase by nine times
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Identify the final products and by-products of
Q22: For the following reaction, pick out the
Q23: Match the chemical descriptors to the following
Q24: Match the chemical descriptors to the species
Q25: Match the chemical descriptors to the species
Q26: For the reaction shown, which one of
Q28: Which is the major product of the
Q29: Match the chemical descriptors to the species
Q30: What is the major product of the
Q31: Which of the following alkyl halides has