Multiple Choice
-tungsten crystallises with a body centred cubic structure where the length of the unit cell is 3.150 Å. Use the diagram to calculate the radius of a tungsten atom. (Hint: The radius of the atom is just half the distance between the two closest atoms.) 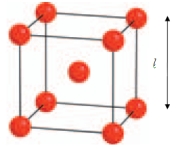
A) 2.692 Å
B) 1.136 Å
C) 1.346 Å
D) 1.575 Å
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Lattice point counting for sphalerite shows that
Q11: Match the material with the bonding type.<br>-platinum
Q12: The ionic radius of an atom can
Q13: Li<sub>2</sub>O crystallises with the antifluorite structure where
Q14: A lattice point on the edge of
Q16: A body centred cubic unit cell is
Q17: Match the material with the bonding type.<br>-S<br>A)
Q18: The coordination number of iodine in cadmium
Q19: Match the value of Born exponent with
Q20: A crystalline solid demonstrates long-range order which