Multiple Choice
Under what conditions would the reaction be most likely to proceed to the product shown at a reasonable rate? (Select one.) 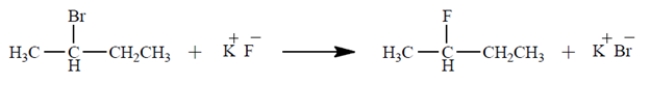
A) in a hydrocarbon solvent such as hexane
B) in ethanol solvent
C) in an ethanol solvent containing some water
D) in very dry dimethyl sulfoxide (DMSO, a polar aprotic solvent) containing a K+-bonding crown ether
E) in dimethyl sulfoxide containing a trace of water and a K+-bonding crown etherf.
in water containing enough acetone to dissolve the alkyl halide, plus a K+-bonding crown ether
Correct Answer:

Verified
Correct Answer:
Verified
Q12: When we say, "sodium ethoxide (Na<sup>+</sup> EtO<sup>−</sup>)
Q13: Circle all possible products for the reaction:<br>
Q14: Give the two organic products of the
Q15: Consider this reaction:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="
Q16: Rank the starting materials in terms
Q18: What are the two products that form
Q19: (a) Provide the structure of the missing
Q20: According to the rate law for the
Q21: Rank these starting materials on their rate
Q22: The rate law for this reaction is