Essay
A mixture of enantiomers of 5-penten-2-ol has an apparent specific rotation of +9.89º mL g−1 dm−1 at temperature = 25 ºC in ether solvent. The sample is known to have an optical purity (= enantiomeric excess) of 67%.
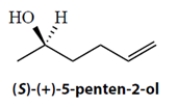 a. What is the specific rotation of the pure S enantiomer under the same conditions? Show your work.
a. What is the specific rotation of the pure S enantiomer under the same conditions? Show your work.
b. What percentage of each enantiomer is present in the mixture? Show your work.
%S enantiomer:_________________; %R enantiomer: ______________
Correct Answer:

Verified
a. The specific rotation of the pure com...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: What is the stereochemistry of the stereoisomeric
Q11: Vasotec, a drug prescribed to treat hypertension,
Q12: The structure of atorvastatin, an important drug
Q13: The structure of pseudoephedrine, a common decongestant,
Q14: Linezolid is an antibiotic used against gram-positive
Q16: Enantiomerically pure (S)-2-bromobutane has a specific optical
Q17: The structure of the beta-blocker drug Nadolol
Q18: Identify whether the molecule is chiral or
Q19: Imagine a hypothetical reaction to make
Q20: Using the templates provided, draw the