Multiple Choice
Consider the reaction free-energy diagram for two reactions that convert reactants (R) into products (P) and select the two correct statements.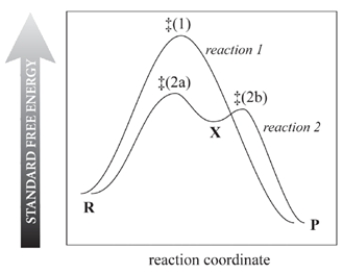
A) Reaction 1 is faster because it occurs in one step.
B) Reaction 2 is faster than reaction 1.
C) Both reactions occur at the same rate at a given concentration of R.
D) X is a transition state of reaction 2.
E) The rate-limiting step of reaction 2 is R ⇌ X.f.
The rate-limiting step of reaction 2 is X ⇌ P.g.
X must be a carbocation.
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Name this compound (including stereochemical configuration where
Q15: Consider this molecule:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider this molecule:
Q16: 14. In each pair, identify the more
Q17: Name the following compound and include the
Q18: Provide an IUPAC name for the compound.<br>
Q20: Give the IUPAC name, including stereochemistry, of
Q21: Consider this reaction. Which reaction would be
Q22: Identify the most stable carbocation:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Identify
Q23: Name the compound. Include an E or
Q24: 14. In each pair, identify the more