Multiple Choice
A multistep reaction A ⇌ B ⇌ C ⇌ D has the free energy-reaction coordinate diagram shown below. Which step of the reaction is rate-limiting in the forward direction?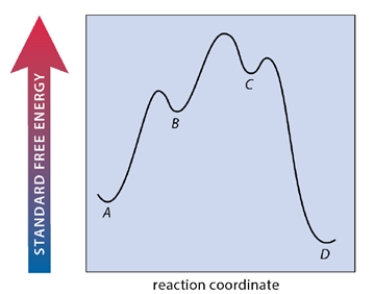
A) the reaction of A to B
B) the reaction of B to C
C) the reaction of C to D
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Complete the reaction by giving the major
Q8: Select the true statements that apply to
Q9: Which of these alkenes has the smallest
Q10: In this pair of compounds, identify the
Q11: Complete the reactions by giving the major
Q13: Provide an IUPAC name for the following
Q14: Name this compound (including stereochemical configuration where
Q15: Consider this molecule:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider this molecule:
Q16: 14. In each pair, identify the more
Q17: Name the following compound and include the