Essay
The nitrogen mustard mustine is toxic to cells because it reacts with two DNA bases on opposite strands of the double helix. Representing the two reacting bases of DNA as R3N: and Rʹ3N: show the products of the reaction of mustine with DNA in a manner that would crosslink the two DNA strands. Your mechanism should account for the very high reactivity of mustine. Show all charges and unshared pairs.
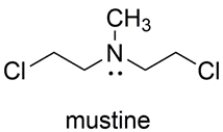
Correct Answer:

Verified
The two DNA bases wi...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Thiophene undergoes electrophilic aromatic substitution (EAS) with
Q16: Name the heterocycle.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Name the
Q17: The heterocyclic base 7-aminopropargyl-7-deaza-2,6-diaminopurine has been incorporated
Q18: Purine is a bicyclic ring found in
Q19: Predict the major organic product for the
Q20: Circle the nitrogen that is more basic
Q21: Give the complementary sequence to the DNA
Q22: Predict the major organic product for this
Q24: Outline a synthesis for the transformation:<br> <img
Q25: Omeprazole is a drug used for the