Essay
Write an arrow-pushing mechanism for the isomerization of enol to cyclohexanone under (a) aqueous acidic conditions and (b) aqueous basic conditions. Show all proton transfer steps in each mechanism and two resonance structures for each intermediate.
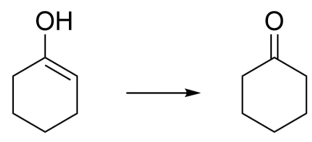
Correct Answer:

Verified
(a) The acidic mechanism starts with pro...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Name the condensation reaction that forms this
Q2: The deuterium exchange of the compound replaces
Q3: Deduce the structure of the starting material
Q5: Can this compound be prepared in good
Q6: One equivalent of ethanethiol reacts with a
Q7: The reaction of lithium aluminum hydride with
Q8: Outline a malonic ester synthesis of the
Q9: Predict the major organic product for the
Q10: Outline a one-step synthesis for the transformation.
Q11: Deduce the structure of the starting material