Short Answer
Palladium tetrakis is shown.
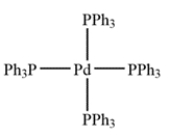 a. Identify the oxidation state of the palladium. ________________
a. Identify the oxidation state of the palladium. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Outline a four-step synthesis to transform 1-butene
Q10: Consider the transition metal complex [Mn(CN)<sub>6</sub>]<sup>3-</sup>.<br>a. Identify
Q11: Predict the major organic products of the
Q12: Which reagent is used in the Stille
Q13: The alkyne can be formed from 1,1-dibromo-3-methylbutane.
Q15: Identify the most acidic compound.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Identify
Q16: Octyl-4-methoxycinnamate is an ingredient found in sunscreens
Q17: Outline a synthesis of this alkyne, using
Q18: Identify the transition-metal catalyzed reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Identify
Q19: Deduce the starting materials that would give