Essay
The diene reacts with HBr to give two isomers A and B (C6H11Br).
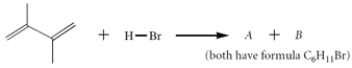 a. Give the structures of A and B.
a. Give the structures of A and B.
b. Give the structure of the carbocation intermediate involved in this reaction. Be sure to show any relevant resonance structures.
c. When compounds A and B are each treated under solvolysis conditions with acetone/water, each compound forms a mixture of the same two alcohols C and D. Give the structures of these two alcohols and explain why both are formed from each alkyl halide.
Correct Answer:

Verified
a. The products are the 1,2- and 1,4-add...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q5: Which compound has UV absorption at the
Q6: Which of these dienes cannot undergo the
Q7: Draw the endo product formed in the
Q8: An arginine residue and a phenylalanine residue
Q9: All but one of these molecules are
Q11: The reaction of an allylic alcohol with
Q12: Draw the major organic product of this
Q13: Which compound or ion has a UV/visible
Q14: Which of these compounds is aromatic?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q15: Determine whether each compound is aromatic, antiaromatic,