Essay
a. Only one of the two diastereomers (formula C12H23OBr) will react with NaOH to form a product X with the formula C12H22O. Identify the reactive diastereomer by circling it and labeling it with the letter A. Draw the product X in its chair conformation.
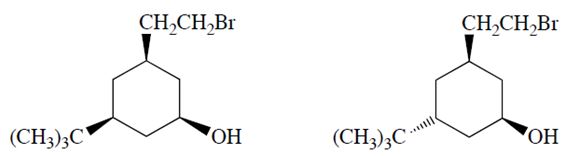 b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
c. Explain why compounds A and B react differently and explain why the formation of X from A is much faster than the formation of Y from B.
Correct Answer:

Verified
a. From the formula it is clear that the...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Propose a synthetic scheme to carry out
Q16: Draw the missing organic product.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q17: In the S<sub>N</sub>2 reaction, sulfur is the
Q18: Provide the missing product and the curved-arrow
Q19: Complete the reaction sequence by providing the
Q20: Provide the missing product. Clearly show stereochemistry
Q21: Outline the synthesis of dicyclopentyl ether using
Q22: Outline a multistep synthesis of the given
Q23: Complete the diagram by supplying structures for
Q25: Benzo[a]pyrene is a carcinogenic polyaromatic hydrocarbon found