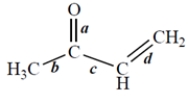
Related Questions
Q1: Consider this structure:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider this
Q2: The lactate ion is a resonance hybrid:<br>
Q3: Which statement is true about molecular orbitals
Q4: The occupied valence orbitals of the chlorine
Q6: Which one of the following statements about
Q7: What is the formal charge on carbon
Q8: In the resonance structures for methyl azide,
Q9: Select the two statements that are true.<br>A)
Q10: According to molecular orbital theory, which
Q11: The atomic orbital that cannot exist is<br>A)