Multiple Choice
Exhibit 2A The structure of ATP with various groups labeled.Group III is the entire phosphate group.
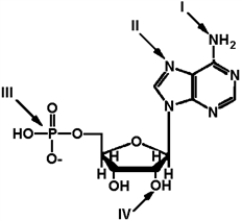
-Refer to Exhibit 2A. Which of the groups could not act as a proton acceptor in a hydrogen bond?
A) I
B) II
C) III
D) IV
E) All can accept a hydrogen in a hydrogen bond.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The water molecule is polar because:<br>A) Electrons
Q26: Exhibit 2B<br>Contains information on the pK's
Q28: Exhibit 2B<br>Contains information on the pK's
Q29: Exhibit 2B<br>Contains information on the pK's
Q38: Which of the following classes of compounds
Q55: Which of the following elements has the
Q57: In a titration of a weak acid
Q61: When does a weak acid buffer best?<br>A)
Q79: What is the pH of an acetic
Q82: The ion product constant for water (K<sub>w</sub>)