Multiple Choice
Phenol and toluene (structures shown below) have similar molecular weights.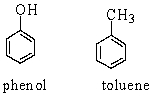 Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C) ?
Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C) ?
A) Toluene is more soluble in water than phenol.
B) Phenol molecules are primarily attracted to one another by London forces.
C) Toluene has greater hydrogen bonding interactions than phenol.
D) Phenol molecules are primarily attracted to one another by hydrogen bonds.
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Provide the IUPAC name for the compound
Q29: The amide containing anti-flea drug Lufenuron is
Q30: The reaction below is an example of
Q31: The structure of amphetamine is shown.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg"
Q32: Determine the major organic product of the
Q34: To which class of compounds would a
Q35: Which of the following lowers the pH
Q36: Which of the following molecules represent trans-
Q37: The correct IUPAC name for the molecule
Q38: The chapter refers to physiological pH, which