Multiple Choice
The equilibrium constant Keq for the reaction of molecular hydrogen and molecular iodine in the gas phase is 54.3 at 430 C.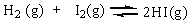 Which of the following statements is true for this reaction at equilibrium?
Which of the following statements is true for this reaction at equilibrium?
A) The reactant concentration is larger than the product concentration.
B) The product concentration is larger than the reactant concentration.
C) The reactant concentration is the same as the product concentration.
D) None of these answer choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The carbonic acid/bicarbonate buffer is important in
Q2: One characteristic of basic solution is that<br>A)
Q3: One liter of a solution is found
Q4: In the equation: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="In the
Q6: Which material would be effective for neutralizing
Q7: Choose the equilibrium constant that indicates the
Q8: Acidic solutions have an H<sup>+</sup> concentration<br>A) greater
Q9: In the Haber process for the production
Q10: The pH of blood is held reasonably
Q11: A buffer is capable of reducing the