Multiple Choice
Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. What does that mean when we are considering pure water? 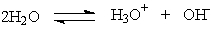
A) More ions exist than water molecules.
B) The majority of the molecules present are in the form of H2O.
C) The amount of water is the same as the amount of the ions present.
D) There will always be more hydronium ions present than water at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q77: A conjugate acid-base pair<br>A) is related by
Q78: 15.00 mL of 0.100 M NaOH is
Q79: Calculate the pH of solution produced by
Q80: The equation:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="The equation:
Q81: What is the [H<sub>3</sub>O<sup>+</sup>] of a solution
Q83: A K<sub>a</sub> can be calculated for some
Q84: The higher the numerical value of an
Q85: A is red, B is colorless, and
Q86: What volume of 0.200 M HCl is
Q87: When a reaction is at equilibrium,<br>A) it