Multiple Choice
The Ka for the reaction of acetic acid and water shown below is 1.8 x 10-5.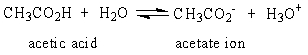 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?
A) there is much more acetic acid than acetate ion
B) there is more acetate ion than acetic acid
C) the concentration of acetate ion is equal to that of acetic acid
D) the pH is lower than pKa of acetic acid
Correct Answer:

Verified
Correct Answer:
Verified
Q50: The greater the hydronium ion concentration, the
Q51: Which of the following are the strong
Q52: A solution in which the concentration of
Q53: The equation:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="The equation:
Q54: Calculate the pH of a 0.01 M
Q56: Excessive use of antacids can lead to<br>A)
Q57: One serious effect of cholera is to
Q58: What is the pH of a solution
Q59: What is the molar concentration of hydronium
Q60: A is red, B is colorless, and