Multiple Choice
The pH of blood is maintained at 7.35-7.45 by the following buffer system: The H2CO3 is produced by the reaction of CO2 with water according to the equation:
The H2CO3 is produced by the reaction of CO2 with water according to the equation: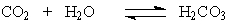 When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
A) The pH will increase.
B) The pH will decrease.
C) The pH will remain the same.
D) It is impossible to predict the effect.
Correct Answer:

Verified
Correct Answer:
Verified
Q29: What is the [H<sub>3</sub>O<sup>+</sup>] of an antacid
Q30: Which of the following is the conjugate
Q31: Acids and bases can react with and
Q32: When stress is applied to a system
Q33: A solution of stomach acid contains 1.21
Q35: What is the concentration of [H<sub>3</sub>O<sup>+</sup>] in
Q36: Compounds that can act as acids and
Q37: Write the equation illustrating how H<sub>2</sub>CO<sub>3 </sub>reacts
Q38: The carbonate/hydrogen carbonate buffer is responsible for
Q39: A sample of blood has a pH