Multiple Choice
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. 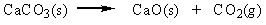
A) single replacement
B) double replacement
C) decomposition
D) synthesis
Correct Answer:

Verified
Correct Answer:
Verified
Q69: Write and balance the equation for the
Q70: For the following organic reactions, identify the
Q71: Consider the burning of methane gas (CH<sub>4</sub>):<br><img
Q72: Figures A and B below represent reaction
Q73: Classify the reaction below as involving synthesis,
Q75: Regarding the following reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Regarding
Q76: The reaction below is an example of
Q77: Which reaction is NOT an oxidation-reduction reaction?<br>A)
Q78: Isooctane, C<sub>8</sub>H<sub>18</sub>, a component of gasoline is
Q79: The percent yield of a reaction is