Multiple Choice
A chemist runs a reaction to prepare bromobenzene, C6H5Br, from the reaction of benzene (C6H6) with bromine(Br2) :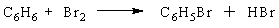 The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
The theoretical yield of the reaction is 87 g of bromobenzene. If 68 g of bromobenzene are obtained, what is the percent yield of the reaction?
A) 93%
B) 81%
C) 78 %
D) 45%
Correct Answer:

Verified
Correct Answer:
Verified
Q49: The reaction below can be classified as
Q50: What will you observe if you mix
Q51: Classify the reaction below as involving synthesis,
Q52: In the reaction below: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="In
Q53: Pentose, C<sub>5</sub>H<sub>10</sub>O<sub>5</sub>, reacts with oxygen to produce
Q55: In the reaction below, _. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg"
Q56: _ is the energy released or gained
Q57: In a synthesis reaction, one compound breaks
Q58: Write the chemical equation for the reaction
Q59: The missing reactant is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="The