Multiple Choice
What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine? 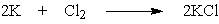
A) 5.2 g KCl
B) 4.9 g KCl
C) 9.9 g KCl
D) 10.1 g KCl
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q76: The reaction below is an example of
Q77: Which reaction is NOT an oxidation-reduction reaction?<br>A)
Q78: Isooctane, C<sub>8</sub>H<sub>18</sub>, a component of gasoline is
Q79: The percent yield of a reaction is
Q80: Which set of coefficients properly balance the
Q82: When properly balanced with whole number
Q83: If the charge on an ion changes
Q84: Barium and sulfur react to produce barium
Q85: Choose the comment that is appropriate for
Q86: In chemical reaction<br>A) the covalent bonds and