Multiple Choice
Two methanol molecules can react in the presence of sulfuric acid to produce dimethyl ether. Calculate the percent yield for this reaction that was run in a laboratory starting with 50 grams methanol and producing 26 grams dimethyl ether. 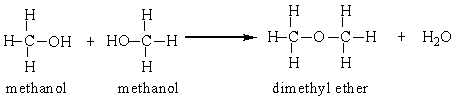
A) 52%
B) 57%
C) 72%
D) 200%
Correct Answer:

Verified
Correct Answer:
Verified
Q39: _ lower the _ of a reaction
Q40: The following equation is an example of
Q41: If ethene (H<sub>2</sub>C=CH<sub>2</sub>) were to react with
Q42: Hydrolysis, hydration and dehydration reactions can take
Q43: The reaction below is an example of
Q45: Aluminum carbonate was once the active ingredient
Q46: Ethene, C<sub>2</sub>H<sub>4</sub>, burns in air according to
Q47: If 2.00 grams of potassium are allowed
Q48: Balance the equation. Tums tablets are composed
Q49: The reaction below can be classified as