Short Answer
Baking soda (NaHCO3) can be used as an antacid. It reacts with and neutralizes stomach acid (HCl). The equation for this reaction is as follows:
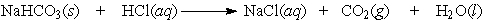 Calculate the mass (in grams) of hydrochloric acid that would be neutralized by 5.0 g of NaHCO3.
Calculate the mass (in grams) of hydrochloric acid that would be neutralized by 5.0 g of NaHCO3.
Correct Answer:

Verified
Correct Answer:
Verified
Q55: In the reaction below, _. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg"
Q56: _ is the energy released or gained
Q57: In a synthesis reaction, one compound breaks
Q58: Write the chemical equation for the reaction
Q59: The missing reactant is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="The
Q61: In the reaction below, which element is
Q62: The catalyst utilized by biological systems for
Q63: How many grams of oxygen (O<sub>2</sub>) are
Q64: Balance the equation and list the coefficients
Q65: In the following reaction, 10.0 moles of