Multiple Choice
The correct line-bond structure for SO42- ion is
A) 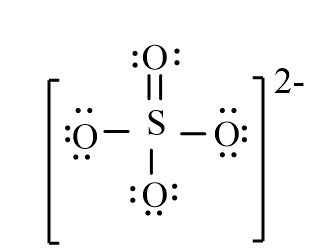
B) 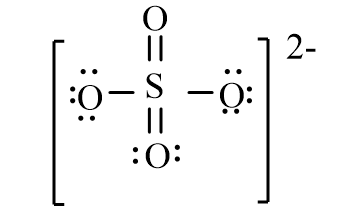
C) 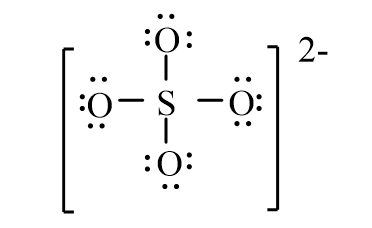
D) 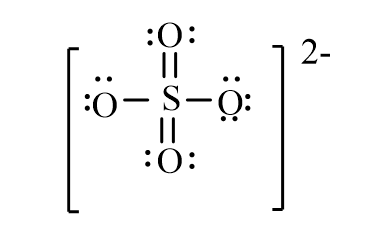
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Draw two ethers that have the formula
Q12: The molecule CH<sub>4</sub> has a tetrahedral
Q13: Dioxygen difluoride whose actual three dimensional shape
Q14: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q15: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q17: Choose the best matching for:<br>-NH<sub>3</sub><br>A) Bent<br>B) pyramidal<br>C)
Q18: Organic compound must contain which of the
Q19: The CaF bond in CaF<sub>2</sub> is nonpolar.
Q20: Sketch the line-bond structure for each molecule
Q21: CH<sub>2</sub>Cl<sub>2</sub> is polar.