Which Atoms in the Following Molecule Would Have - charge Based on the Electronegativities of the Elements
Multiple Choice
Which atoms in the following molecule would have - charge based on the electronegativities of the elements? 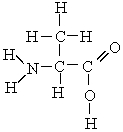
A) H and O
B) O and C
C) C and N
D) N and O
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Choose the best matching for:<br>-CH<sub>4</sub><br>A) Bent<br>B) pyramidal<br>C)
Q58: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q59: Of the two elements, lithium and chromium,
Q60: Which bond is least polar?<br>A) H-F<br>B) H-Cl<br>C)
Q61: What is the shape around C atom
Q63: The strongest noncovalent interaction that can occur
Q64: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q65: Which molecule is nonpolar even though it
Q66: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q67: Sketch the line-bond structure for each molecule