Which Atoms in the Following Molecule Would Have - charge Based on the Electronegativities of the Elements
Multiple Choice
Which atoms in the following molecule would have - charge based on the electronegativities of the elements? 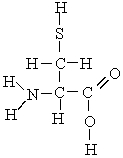
A) H, C, and O
B) N, O, and S
C) C and N
D) N, S, and C
Correct Answer:

Verified
Correct Answer:
Verified
Q65: Which molecule is nonpolar even though it
Q66: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q67: Sketch the line-bond structure for each molecule
Q68: The molecule shown below contains an aldehyde
Q69: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q70: Which of the following bonds can be
Q71: The organic family present in this molecule
Q72: An isotope of fluoromethane, CH<sub>3</sub>F, has been
Q73: The kinds of interactions that exist between
Q75: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose