Multiple Choice
The strongest noncovalent interaction that can occur between two methyl alcohol molecules is ___. 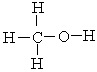
A) dipole-dipole forces
B) hydrogen bonding
C) ion-dipole interaction
D) London force
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Choose the best matching for:<br>-CO<sub>2</sub><br>A) Bent<br>B) pyramidal<br>C)
Q30: Draw two esters with the formula C<sub>5</sub>H<sub>1</sub><sub>0</sub>O
Q31: Hydrocarbons can take part in dipole-dipole interactions.
Q32: Which of the following is the correct
Q33: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose
Q35: Which of the following represents the correct
Q36: Sketch the line-bond structure for each molecule
Q37: This molecule is responsible for the aroma
Q38: Sketch the line-bond structure for each molecule
Q39: Choose the best matching for:<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB10109/.jpg" alt="Choose