Multiple Choice
Using these metal ion/metal standard reduction potentials 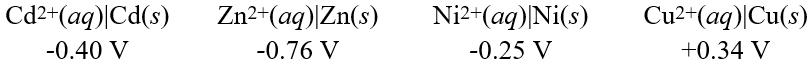 Calculate the standard cell potential for the cell whose reaction is:
Calculate the standard cell potential for the cell whose reaction is:
Cu2+(aq) + Cd(s) Cd2+(aq) + Cu(s)
A) +0.76 V
B) +0.06 V
C) -0.06 V
D) +0.74 V
E) +0.20 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q119: When an aqueous solution of copper sulfate
Q120: Which metal can be prepared by electrolysis
Q121: The Downs cell is used in the
Q122: A galvanic cell is composed of these
Q123: Consider these metal ion/metal standard reduction potentials
Q125: The Faraday constant is equal to the
Q126: How many minutes would be required to
Q127: The equilibrium constant, K<sub>c</sub>, was found to
Q128: A galvanic cell consists of a Cu(s)|Cu<sup>2+</sup>(aq)half-cell
Q129: Which one of the following, when added