Multiple Choice
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 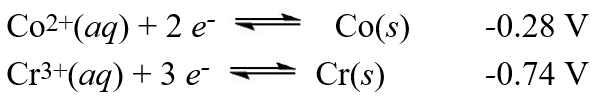 What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
A) -88.8 kJ
B) -178 kJ
C) -266 kJ
D) -295 kJ
E) -590 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q49: Steel objects that are exposed to weather
Q50: If the measured voltage of the cell
Q51: An old classmate who works for Eagle
Q52: How many liters of chlorine gas, measured
Q53: When an aqueous solution of sodium chloride
Q55: During the electrolysis of water, oxygen gas
Q56: When adding half reactions and canceling out
Q57: Semiconductor material is _ with another element,
Q58: A unit of electrical energy is the<br>A)ampere.<br>B)coulomb.<br>C)joule.<br>D)volt.<br>E)watt.
Q59: A galvanic cell is composed of these