Short Answer
Using these metal ion/metal standard reduction potentials 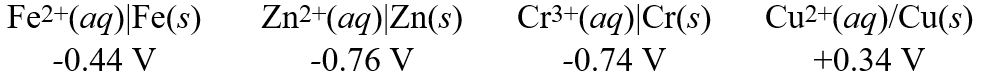 A galvanic cell is composed of these two half-cells:
A galvanic cell is composed of these two half-cells:
Cr3+(aq)| Cr(s)
Cu2+(aq)| Cu(s)
What is the standard reduction potential for the cell reaction of this galvanic cell?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Note: Imaginary elements are used in the
Q3: How many grams of chromium would be
Q4: Which statement is true concerning a galvanic
Q5: A galvanic cell is composed of these
Q6: A unit of electrical charge used is
Q8: The products of the electrolysis of molten
Q9: How many grams of nickel would be
Q10: A galvanic cell consists of an Ag(s)|Ag<sup>+</sup>(aq)half-cell
Q11: The Faraday constant is equal to the
Q12: A galvanic cell is composed of these