Short Answer
Use the standard reduction potentials below to determine the equilibrium constant for the following reaction: 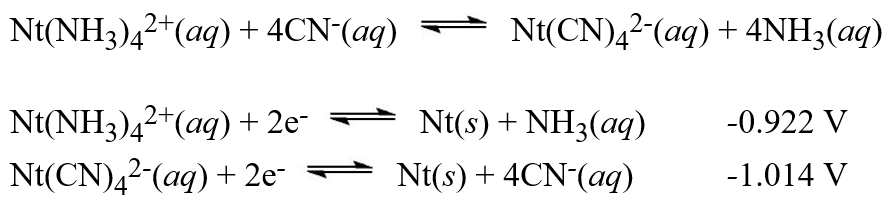 Note that the imaginary element Nt is being used in this reaction.
Note that the imaginary element Nt is being used in this reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: A galvanic cell is composed of these
Q14: How long would it take to deposit
Q15: When an aqueous solution of nickel sulfate
Q16: How many coulombs would be required to
Q17: The standard reduction potentials of Cu<sup>2+</sup>(aq)|Cu(s)and Ag<sup>+</sup>(aq)|Ag(s)are
Q19: The equilibrium constant, K<sub>c</sub>, was found to
Q20: Table of standard electrode potentials <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/.jpg"
Q21: Cations<br>A)are negatively charged ions that result from
Q22: The SI unit for electric current is
Q23: In lithium ion batteries, lithium ions are