Short Answer
The equation for the chemical reaction from which the expression for the solubility product of NtSO4(s)is derived is: NtSO4(s) Nt2+(aq)+ SO42-(aq). Use this equation and the reduction potentials below to help you determine the solubility product of NtSO4 (s). (Note that the imaginary element Nt is being used). 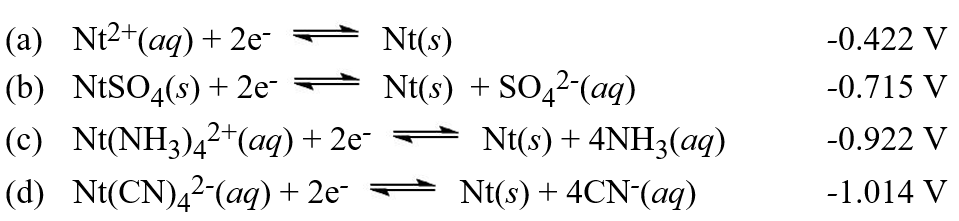
Correct Answer:

Verified
Correct Answer:
Verified
Q133: When an electrical current is passed through
Q134: Consider these metal ion/metal standard reduction potentials
Q135: When fused (molten)sodium chloride is electrolyzed what
Q136: A galvanic cell has two electrodes. Which
Q137: Consider the following reaction: 2Cu<sup>+</sup>(aq)+ Ni<sup>2+</sup> →
Q139: Using these metal ion/metal standard reduction
Q140: Iron objects, such as storage tanks and
Q141: An electrolyte is<br>A)a solid that conducts electrical
Q142: Consider the following reaction: 2Fe<sup>2+</sup>(aq)+ Cu<sup>2+</sup> →
Q143: In the lead storage battery, the electrolyte