Short Answer
Calculate the value of the equilibrium constant, Kc, for the reactionNt(CN)42-(aq)+ SO42-(aq)  NtSO4(s)+ 4CN-(aq)Hint: Use these standard potentials in answering this question. (Note that the imaginary element Nt is being used).
NtSO4(s)+ 4CN-(aq)Hint: Use these standard potentials in answering this question. (Note that the imaginary element Nt is being used). 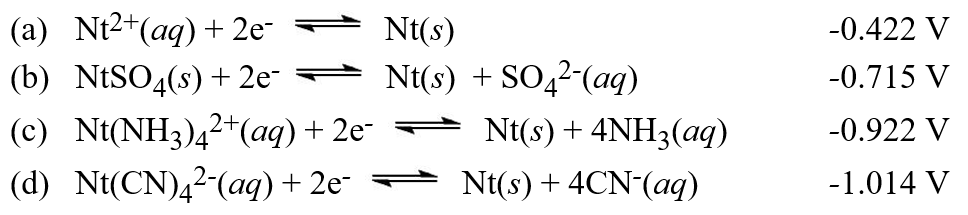
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: The electric charge can be calculated as<br>A)the
Q102: Sketch a galvanic cell with metallic zinc
Q103: One mole of electrical charge contains<br>A)4.184 joules.<br>B)3,600
Q104: The half-reaction that occurs at the
Q105: When an aqueous solution of magnesium sulfate
Q107: A galvanic cell is composed of
Q108: For every oxidation in a chemical process,
Q109: During electrolysis, a current of 3.20 amperes
Q110: A galvanic cell is composed of
Q111: When molten sodium chloride is electrolyzed, a