Multiple Choice
Given the following information: 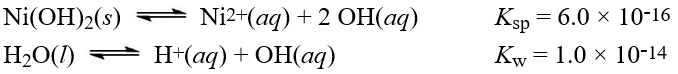 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
A) 1.7 × 1015
B) 6.0 × 10-1
C) 6.0 × 1012
D) 1.7 × 10-13
E) 3.3 × 101
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: Will a precipitate form when 20.0 mL
Q45: Which of the following is the expression
Q46: The solubility of barium carbonate is 14.8
Q47: 200 mL of an aqueous solution contains
Q48: What is the maximum concentration of Mg<sup>2+</sup>
Q50: The sulfide ion is too basic to
Q51: Methylamine, CH<sub>3</sub>NH<sub>2</sub>, is a weak molecular base
Q52: The formation constant for the diammine silver(I)ion
Q53: Metal cations in the chloride solubility group
Q54: The solubility product for Ag<sub>3</sub>PO<sub>4</sub> is: K<sub>sp</sub>