Multiple Choice
Given the following information 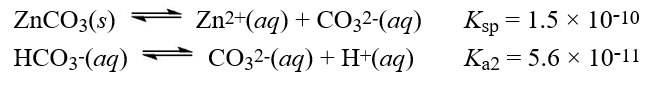 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
A) 8.4 × 10-21
B) 3.7 × 10-1
C) 1.2 × 1020
D) 2.7
E) 2.1 × 10-10
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: What is the solubility, in moles per
Q26: The oxide ion can exist in aqueous
Q27: The solubility of silver phosphate, Ag<sub>3</sub>PO<sub>4</sub>, can
Q28: The solubility product of barium fluoride (BaF<sub>2</sub>)is
Q29: What is the solubility, in moles per
Q31: A solution contains Ba<sup>2+</sup> (1.0 × 10<sup>-3</sup>
Q32: The solubility of silver carbonate, Ag<sub>2</sub>CO<sub>3</sub>, in
Q33: The K<sub>sp</sub> value for barium chromate is
Q34: The solubility of lead iodide is 578
Q35: A precipitate will form when a solution