Short Answer
A solution of chloroform (CHCl3)and acetone ((CH3)2CO)exhibits a negative deviation from Raoult's law similar to that shown on the diagram below. What does this tell us about the solution with respect to it being an ideal solution and the strength of the interactions between acetone and chloroform? This result shows us that the solution is ________ ideal and the interactions between chloroform-chloroform and acetone-acetone are ________ than the interactions between chloroform/acetone. 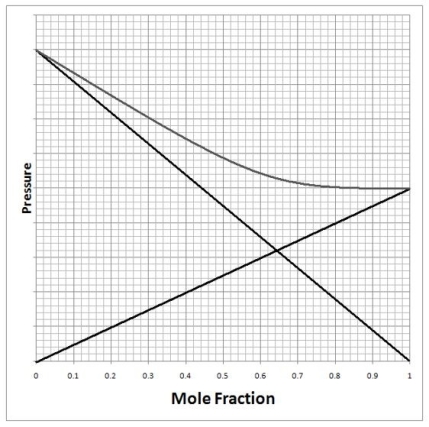
Correct Answer:

Verified
Correct Answer:
Verified
Q24: KBr has a lattice energy of
Q25: Which can be used to calculate the
Q26: Pure glacial acetic acid, HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>, has a
Q27: Liquids that are mutually miscible possess intermolecular
Q28: A solution in a beaker has some
Q30: One driving force toward formation of homogeneous
Q31: An aqueous solution of glycerol, C<sub>3</sub>H<sub>8</sub>O<sub>3</sub> is
Q32: The solubility of O<sub>2</sub> in water is
Q33: Which aqueous solution will have the lowest
Q34: Which aqueous solution should have the highest