Essay
A phase diagram, like the one below, for an aqueous solution shows a shift in the solid/liquid and liquid/gas phase transitions but there is not a shift in the sublimation line between solids and gases. Explain what causes the shift in the other transitions and why this does not change a shift in the solid/gas transition.Hint: Think about the difference in vapor pressure between a solution and a pure substance. 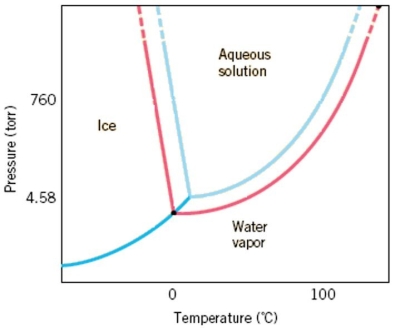
Correct Answer:

Verified
For the solid/liquid transition the solu...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: Concerning the solvation step of the solution
Q18: An aqueous solution that would cause red
Q19: What is the expected freezing point of
Q20: A solution is made by dissolving 48.07
Q21: The vapor pressure of a solution containing
Q23: The CO<sub>2</sub> gas sealed inside a carbonated
Q24: KBr has a lattice energy of
Q25: Which can be used to calculate the
Q26: Pure glacial acetic acid, HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>, has a
Q27: Liquids that are mutually miscible possess intermolecular