Multiple Choice
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 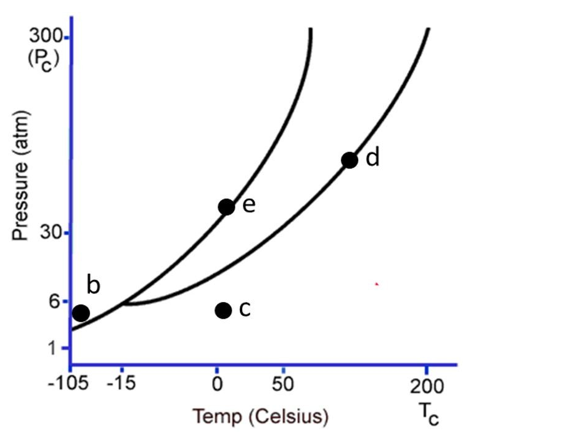
-At the temperature and pressure of point d, which statement below is true?
A) The substance will sublime.
B) There will be an equilibrium between the solid phase and the gaseous phase.
C) Vaporization and deposition will take place simultaneously.
D) Condensation and evaporation will take place simultaneously.
E) The substance will be a supercritical fluid.
Correct Answer:

Verified
Correct Answer:
Verified
Q179: An unknown solid is hard, but malleable
Q180: The unit cell below is best described
Q181: The vapor pressure of ethanol is 400
Q182: A cylinder contains a liquid which is
Q183: Will a nonpolar molecule will have the
Q184: Which compound will have the weakest intermolecular
Q185: Arrange these compounds in order of increasing
Q187: Given the phase changes: condensation, freezing, fusion,
Q188: Which of the following liquids, at the
Q189: For small molecules of comparable molecular weight,