Multiple Choice
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 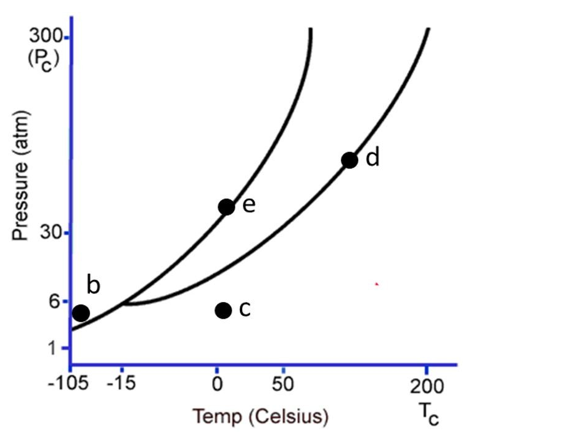
-At the temperature and pressure of point e, which statement below is true?
A) The substance will sublime.
B) There will be an equilibrium between the solid phase and the gaseous phase.
C) Vaporization and deposition will take place simultaneously.
D) Melting and freezing will take place simultaneously.
E) Melting and vaporization will take place simultaneously.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: A substance has a normal boiling point
Q3: Keshon has an equation that gives the
Q4: Determine the shift in equilibrium that would
Q5: How many atoms are contained in one
Q6: X-ray radiation at a wavelength of 1.542
Q7: A new compound, voronium oxide, has been
Q8: Given the following substances and their normal
Q9: All substances have London forces.
Q10: Which one of the following substances is
Q11: Arrange these compounds in order of increasing