Multiple Choice
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 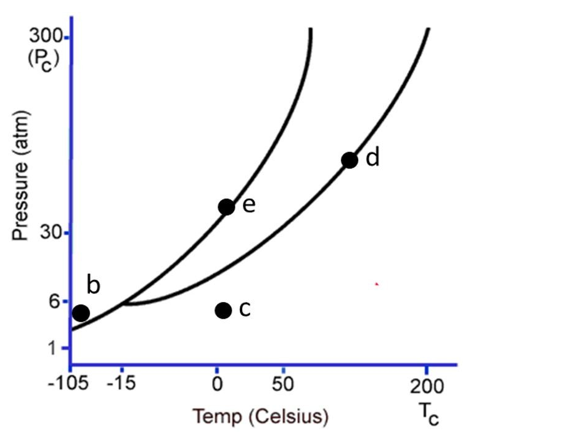
-Starting at the temperature and pressure of b, if the temperature is increased at constant pressure, ultimately
A) the substance will sublime.
B) the substance will undergo fusion.
C) the substance will undergo deposition.
D) the substance will freeze.
E) the substance will be a supercritical fluid.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Describe the essential features of a hydrogen
Q34: Which of the following would be expected
Q35: How many atoms are contained in one
Q36: A unit cell of sodium chloride (face-centered
Q37: Which set of properties below best describes
Q39: The property that measures or describes the
Q40: The following questions refer to the diagram
Q41: The following questions refer to the phase
Q42: A unit cell of sodium chloride (face-centered
Q43: Which set of properties below best describes