Multiple Choice
The following questions refer to the phase diagram below. Gas, liquid, and solid phases are all present, but not labeled. 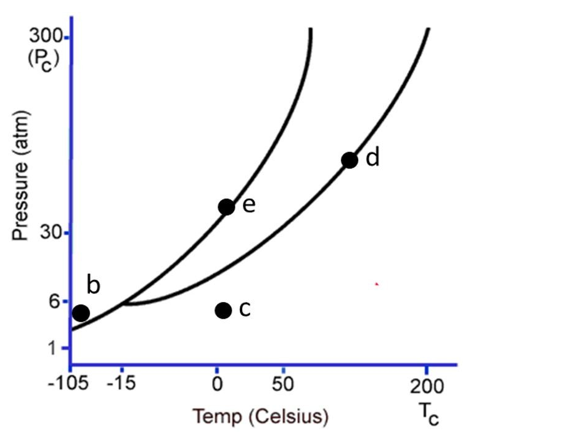
-Starting at the temperature and pressure of c, if the pressure is increased at constant temperature, ultimately
A) the substance will sublime.
B) the substance will undergo fusion.
C) the substance will undergo deposition.
D) the substance will freeze.
E) the substance will undergo condensation.
Correct Answer:

Verified
Correct Answer:
Verified
Q100: What other molecular factors determine the viscosity
Q101: What is the difference between intermolecular interactions
Q102: What is the mass of a sample
Q103: Arrange these compounds in order of increasing
Q104: The term "London forces"is a synonym for<br>A)ion-ion
Q106: The following questions refer to the basic
Q107: The heat capacity of liquid water is
Q108: How many atoms are contained in one
Q109: Which property of water allows some bugs
Q110: Which compound should have the lowest boiling