Multiple Choice
The following questions refer to the phase diagram below. 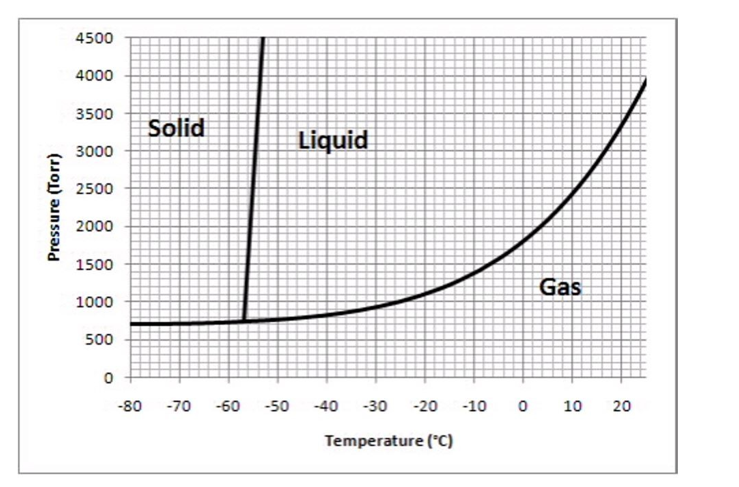
-What phase should this substance exist as, at a pressure of 1500 torr and a temperature of -20°C?
A) solid
B) liquid
C) gas
D) supercritical fluid
E) unable to tell
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q154: Which covalent compound will exhibit hydrogen bonding
Q155: A substance with a high boiling point
Q156: The unit cell below is best described
Q157: The ease with which the electron cloud
Q158: The strongest intermolecular forces between molecules of
Q160: What lies in the geometric center of
Q161: Which compound should have the highest boiling
Q162: Silicon carbide, which has the empirical formula
Q163: Which type of unit cell has lattice
Q164: Osmium tetroxide, OsO<sub>4</sub>, forms a crystal that