Short Answer
The following questions refer to the basic phase diagram below with gas, liquid, and solid phases present and labeled. 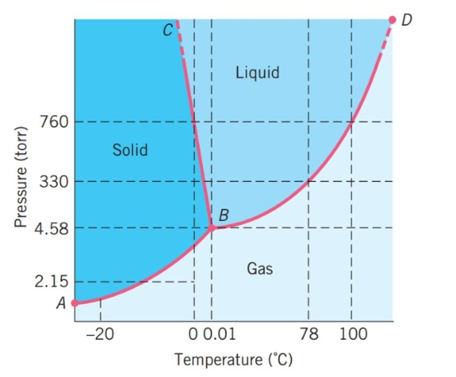
-If the substance starts at a pressure of 330 torr and -20°C and is heated at constant pressure to 20°C, what phase transition will occur?
Correct Answer:

Verified
Correct Answer:
Verified
Q63: Portions of how many different atoms are
Q64: The following questions refer to the diagram
Q65: Viscosity is determined only by the intermolecular
Q66: Supercooling is defined as<br>A)the extremely rapid cooling
Q67: Benzene, C<sub>6</sub>H<sub>8</sub>, has an enthalpy of fusion
Q69: When a liquid undergoes a change of
Q70: What are the characteristics that distinguish solids,
Q71: When a gas undergoes a change of
Q72: Explain how you could cause pure water
Q73: A solid with a molecular weight of