Short Answer
The following questions refer to the basic phase diagram below with gas, liquid, and solid phases present and labeled. 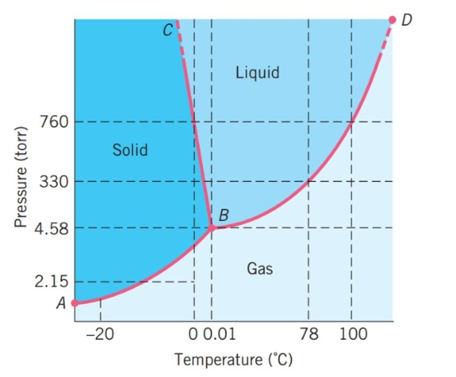
-Above ________ °C, it is not possible for a liquid phase of this substance to exist.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: The following questions refer to the phase
Q42: A unit cell of sodium chloride (face-centered
Q43: Which set of properties below best describes
Q44: The critical temperature of a substance is<br>A)always
Q45: 1,3,6,8-Tetramethylnaphthlene is a white crystalline solid which
Q47: The following questions refer to the diagram
Q48: A unit cell of sodium chloride (face-centered
Q49: p-Xylene, C<sub>8</sub>H<sub>10</sub>, has an enthalpy of fusion
Q50: Which set of properties below best describes
Q51: Which of the following substances has the