Multiple Choice
The van der Waals equation of state for a real gas is 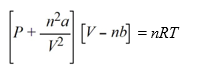 In this equation, the van der Waals constant, b, represents a correction for
In this equation, the van der Waals constant, b, represents a correction for
A) a positive deviation in the measured value of V from that for an ideal gas due to the finite volume of space occupied by molecules of a real gas.
B) a negative deviation in the measured value of V from that for an ideal gas due
To the finite volume of space occupied by molecules of a real gas.
C) a positive deviation in the measured value of V from that for an ideal gas due to the attractive forces between the molecules of a real gas.
D) a negative deviation in the measured value of V from that for an ideal gas due to the attractive forces between the molecules of a real gas.
E) a positive deviation in the measured value of V from that for an ideal gas due to the finite mass of the molecules of a real gas.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Air pressure at the top of a
Q2: A gas mixture is 50.00% helium and
Q3: Whipped cream in a can contains whipped
Q4: Even though the average speed of a
Q5: Air pressure at the bottom of a
Q7: Why do a person's ears "pop"when changing
Q8: What are the values for standard temperature
Q9: A sample of a gas occupies a
Q10: The product, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/.jpg" alt="The product,
Q11: A sample of hydrogen gas is placed