Multiple Choice
The van der Waals equation of state for a real gas is 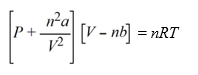 At what pressure will 1.00 mole of CH4 be in a 10.0 L container at 298 K, assuming that CH4 is a real gas?(van der Waals constants for CH4 are a = 2.253 L2 atm mol-2, b = 0.04278 L mol-1)
At what pressure will 1.00 mole of CH4 be in a 10.0 L container at 298 K, assuming that CH4 is a real gas?(van der Waals constants for CH4 are a = 2.253 L2 atm mol-2, b = 0.04278 L mol-1)
A) 2.43 atm
B) 2.28 atm
C) 2.51 atm
D) 24.5 atm
E) 0.440 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q122: An open-end mercury manometer was constructed from
Q123: For a substance that remains a gas
Q124: The density of a gas sample was
Q125: In order for a gas to be
Q126: Mountain climbers often need to take tanks
Q128: Which of the following is a condition
Q129: A sealed glass container contains partial pressures
Q130: A gas sample is attached to a
Q131: A sample of a gas in a
Q132: A sample of a gas occupies a