Multiple Choice
The van der Waals equation of state for a real gas is 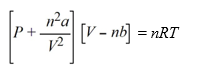 At what pressure will 1.00 mole of NH3 be in a 10.00 L container at 298 K, assuming NH3 is a real gas?(van der Waals constants for NH3 are a = 4.170 L2 atm mol-2, b = 0.03707 L mol-1)
At what pressure will 1.00 mole of NH3 be in a 10.00 L container at 298 K, assuming NH3 is a real gas?(van der Waals constants for NH3 are a = 4.170 L2 atm mol-2, b = 0.03707 L mol-1)
A) 2.03 atm
B) 20.3 atm
C) 2.41 atm
D) 24.1 atm
E) 2.47 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: A certain gas behaves as an ideal
Q107: A sample of a gas occupies a
Q108: The average speed at which a nitrogen
Q109: How many liters of pure oxygen gas,
Q110: A gas sample occupies a volume of
Q112: Gases can have negative volumes when the
Q113: A gas sample containing 0.2820 moles of
Q114: What is the total pressure exerted by
Q115: A sealed glass container contains partial pressures
Q116: A sample of a gas occupies a