Short Answer
What is the gas pressure of the gas sample hooked up to the mercury manometer shown below if the atmospheric pressure is 756 mmHg? 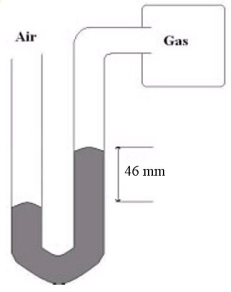
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q134: Calculate the density of Br<sub>2</sub> gas at
Q135: What is the mole fraction of methane
Q136: A pressure measured using a mercury barometer
Q137: A closed-end manometer was constructed from a
Q138: If container "A"is occupied by 1.00 mole
Q140: A gaseous element has a density of
Q141: What is the total pressure exerted by
Q142: A sample of a gas in a
Q143: 8.25 g of liquid hexane (C<sub>6</sub>H<sub>14</sub>)is introduced
Q144: A gas sample weighing 8.280 grams occupies